By Happiness Inuetinyan
Introduction
Understanding the thermophysical properties of multicomponent fluid mixtures is crucial for industrial applications such as carbon capture and storage (CCS), enhanced oil recovery (EOR), and supercritical CO₂ processing. In our recent study, published in the Journal of Chemical & Engineering Data, we investigated the equilibrium thermophysical properties of ternary mixtures containing carbon dioxide (CO₂), cyclohexanol, and toluene. This research fills a critical gap in the literature, as previous studies have primarily focused on binary CO₂ systems, leaving ternary systems largely unexplored.
Key Findings
Phase Behavior
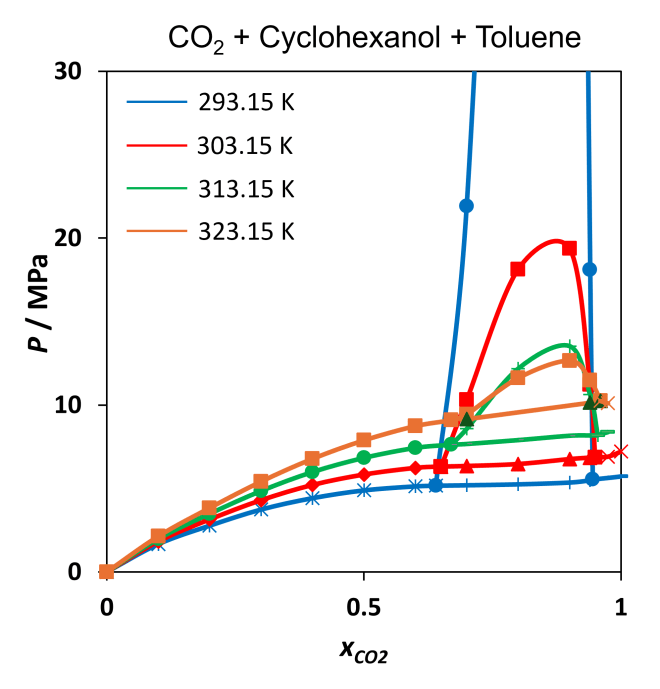
Using a high-pressure variable-volume cell, we examined the phase behaviour of CO₂ + cyclohexanol + toluene mixtures across a temperature range of 293.15–353.15 K and pressures up to 30 MPa. Key observations include:
- Liquid-Liquid Immiscibility: At CO₂ mole fractions exceeding 0.6 mol/mol, the mixture exhibited liquid-liquid immiscibility, particularly at lower temperatures (293.15–313.15 K).
- Type III Phase Behaviour: The system displayed complex phase transitions, including liquid-liquid-vapour (LLV) equilibria, characteristic of Type III behaviour in the Scott and Van Konynenburg classification.
- Pressure-Temperature Trends: Higher CO₂ concentrations increased the pressure required for phase transitions, resulting in a distinctive “U-shaped” curve in the pressure-temperature diagrams for CO₂-rich mixtures.
Density Measurements
Density data were collected for both binary (cyclohexanol + toluene) and ternary (CO₂ + cyclohexanol + toluene) mixtures using a vibrating U-tube densimeter. Notable results include:
- Temperature Dependence: At lower temperatures (293.15–303.15 K), density increased with rising CO₂ content. However, at higher temperatures (333.15–353.15 K), density peaked at intermediate CO₂ mole fractions before declining.
- Simulation Agreement: Molecular simulations predicted density trends with an average deviation of 1.7% from experimental data, validating the reliability of the computational models.
Henry’s Law Constant
Molecular dynamics simulations estimated the Henry’s law constant for CO₂ in the binary cyclohexanol + toluene mixture. The results showed:
- Temperature Sensitivity: The Henry’s law constant increased with temperature, peaking at 533.15 K before decreasing.
- Comparative Insights: While experimental data for the ternary system were scarce, simulations aligned well with existing literature for CO₂ solubility in pure toluene and cyclohexanol.
Conclusion
This study bridges a significant gap in the literature by offering comprehensive experimental and simulation data for the CO₂ + cyclohexanol + toluene system. The results highlight the complex interplay of temperature, pressure, and composition in determining phase behaviour and density, paving the way for further research into transport properties and broader industrial applications.
For more details, check out the full paper here.